Creative Proteomics has extensive knowledge in protein post-translational modification analysis. With a professional team and advanced mass spectrometry facilities, we can provide you with a one-stop protein disulfide bond formation identification service from experimental design to result from delivery to accelerate your research progress.
Disulfide bonds are post-translational modifications of proteins formed by cysteine residues in proteins. The formation of this covalent bond comes from the free sulfhydryl group of cysteine, mainly catalyzed by enzymes. In the tertiary structure of proteins, disulfide bonds can firstly ensure the normal folding of the protein, secondly, enable the protein to form the correct structural subtype, and thirdly, stabilize the higher-order confirmation to ensure normal biological functions. In protein quaternary structure, the reduction of disulfide bonds can also cause changes in the activity of certain proteins. Disulfide bonds are very important in living organisms, and the precise analysis of the formation of disulfide bonds is of great significance for subsequent research.
Service Content of Protein Disulfide Bond Formation Identification
In the protein disulfide bond formation identification service, Creative Proteomics uses our advanced mass spectrometry facilities to identify and quantify protein disulfide bonds, find disulfide bond sites, and identify disulfide bond structure types. In this service, we perform reductive alkylation of free sulfhydryl groups by NEM, followed by blocking of broken disulfide bonds with IAM, and enzymatic separation of substrates in gel or solution. Finally, we use our professional MS analysis to obtain the complete structural information of the disulfide bond. Our protein disulfide bond analysis solutions will give you all the relevant information about protein disulfide bonds.
Mass Spectrometry Facilities and Projects
- Sciex Triple TOF 6600 Mass Spectrometer
- Thermo Scientific™ Orbitrap Fusion™ Tribrid™ Mass Spectrometer
- Thermo Scientific™ Orbitrap Fusion™ Lumos™ Tribrid™ Mass Spectrometer
- Thermo Scientific™ Q Exactive™ HF Hybrid Quadrupole-Orbitrap Mass Spectrometer
Requirements for Samples
We accept many types of samples to meet your various needs.
- Cell sample: the sample cell content should be no less than 107.
- Microbial samples: the dry weight of yeast and microorganisms should be > 200 mg.
- Solution (target protein): total target protein > 50 μg, target protein concentration > 80%.
- Solution (large-scale mixed protein): total protein > 1 mg, protein concentration > 1 μg/μL.
- Tissue samples: animal tissue samples > 1 g, blood samples >1 mL, serum samples > 0.2 mL, urine samples > 2 mL, plant tissue samples > 200 mg.
- Please provide the specific concentration, volume, preparation time, and source of each sample. And inform the sample information as well as the control and experimental samples (if there is a group, describe the group information in detail.)
Deliverables
- Experimental steps
- Relevant mass spectrometry parameters
- Details of the identified phosphorylation sites
- Mass spectrometry images
- Raw data
Our Advantages
- State-of-the-art mass spectrometry facilities and strict quality control standards.
- The professional experimental team can accurately and quickly identify the peptide site of the disulfide bond.
- The project quotation is clear, and real-time feedback of the experimental progress is provided for you throughout the process.
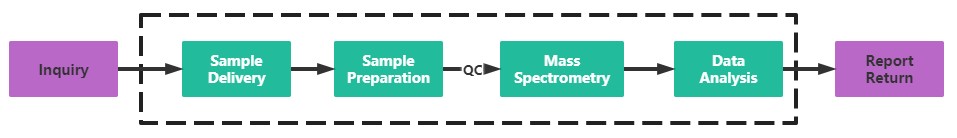
Service Process
Creative Proteomics has long been committed to providing high-quality mass spectrometry identification service and has rich experience and profound insights into the identification of protein disulfide bonds. We look forward to providing you with a professional protein disulfide bond formation identification service. If you are interested in our service, please contact us today for expert support.
The service is for research only, not for clinical use.